Understanding how cerebral blood flow is controlled is essential for many areas of brain research – but what happens during pathological states has always been less clear. Researchers based in Lyon have now used functional ultrasound (fUS) equipment from Iconeus to investigate the timing and distribution of 0.1 Hz neurovascular ‘travelling waves’ in a rat model of inflammation. They concluded that such waves are a major contributor to cerebral hemodynamics in such situations, and suggest that imaging them during disease states could provide information that complements traditional measurements.
The link between neuron activity and increased blood flow (hyperemia) is a valuable paradigm in brain science, with the principal mechanism in the healthy brain being activity in the neurons causing the surrounding glial cells to release vasoactive substances. However, it is also believed that during pathological conditions, other processes may take on a significant role, with one of these being vasomotion – a rhythmic oscillation in vessel diameter at a frequency of about 0.1 Hz.
Vasomotion is believed to originate from spontaneous contraction/relaxation of smooth muscle cells (SMCs) that surround the blood vessels, with the low frequency naturally synchronized to the activity of neurons in order to meet their energy demands. However, the spatiotemporal dynamics of vasomotion, as well as how it relates to neuronal and glial functions, remains unclear. In addition, up to now there have been few studies examining how vasomotion operates during pathological conditions.
Using fUS to investigate vasomotion in the rat
These questions have now been explored by Benjamin Vidal and colleagues at the Université Claude Bernard in Lyon, along with collaborators at Theranexus and the Hospices Civils de Lyon. For the first time, they have used fUS to investigate various aspects of cerebral vasomotion in rats under conditions of inflammation, which is a feature of the vast majority of brain diseases.
In their multi-part study published in eBiomedicine, Vidal and team used an intracerebral injection of lipopolysaccharide (LPS) to induce inflammation in rats that had previously undergone a skull-thinning procedure to ensure efficient transmission of the fUS ultrasound signals. An hour after this injection, an Iconeus One probe was used to monitor the cerebral hemodynamics in the coronal plane, both in the resting state and with visual stimulation. The fUS protocol was repeated 48 hours later, and was followed by an ultrasound localization microscopy (ULM) protocol, again with Iconeus One, using microbubbles to achieve ultra-high-resolution visualization of the vasculature.
To complement this main part of the study, several other experiments were also carried out, including controls, resting-state-only fUS acquisitions at 0, 1 and 7 days on freely moving animals, and post-mortem studies to assess glial cell reactivity.
‘Travelling waves’ in the brain vasculature
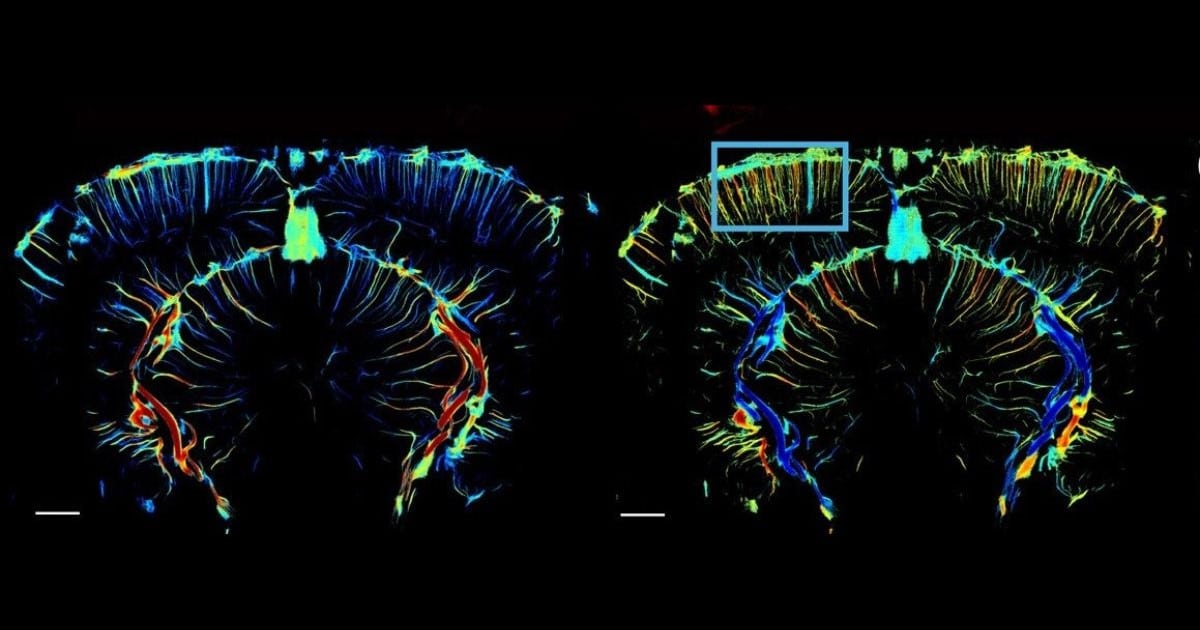
The main finding by the team is that, 24–48 hours after injection of LPS, cerebral hemodynamic activity shifts towards an oscillatory state. This state, they say, is “characterised by quasi-sinusoidal, high-amplitude oscillations at ~0.1 Hz, that locally propagate through the vascular tree” – with this propagation occurring at 0.1–0.8 mm/s, and largely taking place in the cortex.
Example fUS images from the mouse study, over a period of 0–7 weeks, showing the change in cerebral blood volume (CBV) following whisker stimulation in (A) a control animal and (C) an animal treated with cuprizone and exhibiting the demyelination characteristic of MS; note the increased response at 3 weeks and 5 weeks. Reproduced from Imaging Neuroscience and published under CC BY 4.0.
The observation that these ‘travelling waves’ are localized supports a conclusion of earlier workers, namely that they do not originate from blood pressure fluctuations. Instead, it is concluded that they instead represent true vasomotion, likely deriving from intracellular calcium fluctuations in the smooth muscle cells.
This was corroborated by the ULM experiment, which (as well as enabling identification of pial vessels, arterioles and venules) showed that there were no major changes in absolute blood flow velocity. Furthermore, the supplementary studies suggested that both microglia and astrocytes are involved in this enhancement of vasomotion, and that functional connectivity and functional hyperemia were also increased.
Representative raw (left) and processed (right) data showing cerebral blood volume changes pixel-by-pixel across 100 consecutive images, during resting-state in an awake animal, 48 h after LPS injection.
fUS: Broad coverage, high sensitivity
The authors found that, particularly in the LPS-treated animals, the travelling waves could propagate surprisingly long distances through the cortex, for example from the ventral hippocampus to the cortex in the contralateral hemisphere. Such phenomena, they say, were “easily detected” using fUS, in contrast to optical techniques used by some previous workers, which are limited to imaging surface regions.
Another benefit of fUS that was of value in this study, say Vidal and colleagues, was the ability of fUS to “map cerebral blood volume changes at a mesoscopic scale with higher sensitivity than fMRI”, which enabled them to track the progress of individual travelling waves. They also mention the technique’s capability to achieve imaging in freely moving rodents – which here was employed in the supplementary part of the study, using a head plate designed by Iconeus staff.
The contribution of vasomotion to cerebral hemodynamics
In conclusion, this work highlights vasomotion as what the authors say is a “major component” of cerebral hemodynamics during inflammation. This, they point out, has implications for how researchers interpret data obtained on functional neuroimaging in patients with these conditions, particularly in light of the other major morphological, functional, and molecular changes that take place during neuroinflammation.
For example, rather than attributing blood flow changes in resting-state or functional hyperemia solely to neuronal activity, researchers may be able to implicate part of the response to vasomotion driven by glial cell activity. They conclude by pointing out that such low-frequency vasomotion, detected using fUS, could serve as a potential biomarker of glial reactivity in future research, and be an important complement to traditional measurements.
Ludovic Lecointre, Pharm.D., CEO and co-founder of Iconeus, said: “This is a very interesting and thorough piece of work that sheds light on a feature of neuroinflammation models that is challenging to study using conventional imaging tools. We’re of course pleased that the sensitivity and depth-of-field of Iconeus One have proven so useful, and look forward to more systematic, brain-wide evaluations of these travelling waves, which Dr Vidal and colleagues highlight as a promising avenue of future research”.
Reference:
M. Pereira, M. Droguerre, M. Valdebenito, L. Vidal, G. Marcy, S. Benkeder, P. Marchal, J.-C. Comte, O. Pascual, L. Zimmer and B. Vidal, Induction of haemodynamic travelling waves by glial-related vasomotion in a rat model of neuroinflammation: Implications for functional neuroimaging, eBiomedicine, 2025, 116: 105777, http://doi.org/10.1016/j.ebiom.2025.105777