Three peer-reviewed studies validate the use of fUS in rats and mice as a tool for neuropharmacological studies (‘pharmaco-fUS’), offering the prospect of an improved understanding of the mechanism of action of drugs in the brain, and potentially accelerating the development of new drugs targeting neurological disorders.
Three proof-of-concept studies suggest that functional ultrasound (fUS) imaging is a powerful approach for studying the effects of pharmacological agents on the brain, without the complication of anesthesia seen in fMRI studies.
Investigating the effects of scopolamine, donepezil, mefloquine and atomoxetine
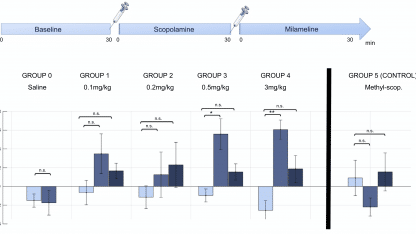
In the first study[1], researchers working in collaboration with Iconeus’ founders showed that scopolamine, a major preclinical drug for modelling the effects of Alzheimer’s disease, leads to time- and dose-dependent changes in cerebral functional connectivity in awake mice. A machine-learning approach was used to extract a robust pharmacological score or ‘fingerprint’ of the drug’s effects on the brain from a relatively small cohort of mice. The pharmacological relevance of the scopolamine score was demonstrated by reversal through the antagonist milameline and the peripherally-restricted methylscopolamine.
The second and third studies, both from the pharmaceutical company Theranexus, demonstrated how fUS can be useful for drug development.
In the first of these[3], the authors investigated the hemodynamic response of the brain when administered with donepezil (a potent AChE inhibitor, used worldwide to alleviate cognitive symptoms in Alzheimer’s disease) and/or mefloquine (a new drug targeting connexins, the proteins involved in astrocyte network organization). They showed that although administration of either drug on its own at low dose had only very limited effects on the signal compared to the baseline, combining them produced marked hemodynamic effects in the hippocampus, in line with previously published behavioral data demonstrating a synergic interaction between both drugs. These results confirmed the potential of the combined treatment and provided new insights on how both drugs could interact.
In the other study from Theranexus[3], the authors studied the effect of atomoxetine (a potent norepinephrine re-uptake inhibitor and non-stimulant treatment for ADHD) in anesthetized rats at doses of 0.3, 1 and 3 mg/kg. Dose-dependent temporal variations of the cerebral blood flow were observed, particularly in brain regions involved in vision.
Investigating the functional connectivity in the brains of neonates
Now, in a paper in Nature Communications, authored by a team including Iconeus staff members, we’ve described how we recorded the brain activity of neonates at rest. We then used three methods to assess the functional connectivity, each exploiting different characteristics of the data and providing complementary information.
From this, we were able to extract functional connectivity patterns, and also study how they evolved over time, and how often each pattern occurred. This is a notable advance, because although dynamic functional connectivity has been studied in adults using fMRI, very little data has been obtained on neonates so far. Interestingly, these initial results obtained using fUS in a small cohort suggest a significant difference between term and preterm babies.
‘Pharmaco-fUS’ – Improving understanding of drug mechanisms of action
Together, these three studies demonstrate that using fUS for neuropharmacological studies could improve our understanding of the mechanism of action of drugs in the brain, and might accelerate the development of new drugs targeting neurological disorders. fUS does not require the animals to be anesthetized, and therefore yields a quantitative assessment of brain functions without anesthesia-induced bias. In addition, although fUS has currently been applied to cross-sectional (2D) images of the rodent brain, volumetric (3D) imaging is in development, and this is expected to enable drug effects to be assessed in the entire rodent brain within a single imaging session.
References:
[1] C. Rabut, J. Ferrier, A. Bertolo, B. Osmanski, X. Mousset, S. Pezet, T. Deffieux, Z. Lenkei and M. Tanter, Pharmaco-fUS: Quantification of pharmacologically-induced dynamic changes in brain perfusion and connectivity by functional ultrasound imaging in awake mice, NeuroImage, 2020 doi:10.1016/j.neuroimage.2020.117231
[2] B. Vidal, M. Droguerre ,M. Valdebenito, L. Zimmer, M. Hamon, F. Mouthon and M. Charvériat, Pharmaco-fUS for characterizing drugs for Alzheimer’s disease – The case of THN201, a drug combination of Donepezil plus Mefloquine, Frontiers in Neuroscience, 2020, 14: 835.
[3] B. Vidal, M. Droguerre, L. Venet, L. Zimmer, M. Valdebenito, F. Mouthon and M. Charvériat, Functional ultrasound imaging to study brain dynamics: Application of pharmaco-fUS to atomoxetine, Neuropharmacology, 2020, 179: 108273.