Real-time fUS imaging of human brain activity through an acoustic window
: An article in the journal Science Translational Medicine describes the first use of an ‘acoustic window’ in a human subject to achieve high-resolution fUS imaging.
A study by Californian researchers has demonstrated for the first time how to use an acoustically transparent cranial window to achieve real-time imaging of human brain activity. We summarize their work, and consider what this major advance could mean for the future of fUS.
In the relatively short time since the development of practical systems for carrying out functional ultrasound (fUS), the technique has proved exceptionally useful for investigating brain function in preclinical models. But to achieve the full potential of fUS, this technology needs to be translated to the clinical arena – and that is a challenge because the thickness of the human skull hampers the effective transmission of the high-frequency ultrasound signals needed to achieve high spatial resolution.
One of the most promising solutions to this problem is using an ‘acoustic window’ in the skull. This takes the form of a sheet of low-density polymer, transparent to ultrasound waves, that is used to replace a section of the skull. Up to now, this has only been carried out in mice and rats, but new work, led by researchers from Caltech, Pasadena, the University of Southern California, and the University of Riverside, shows for the first time how this can be translated to a human subject.
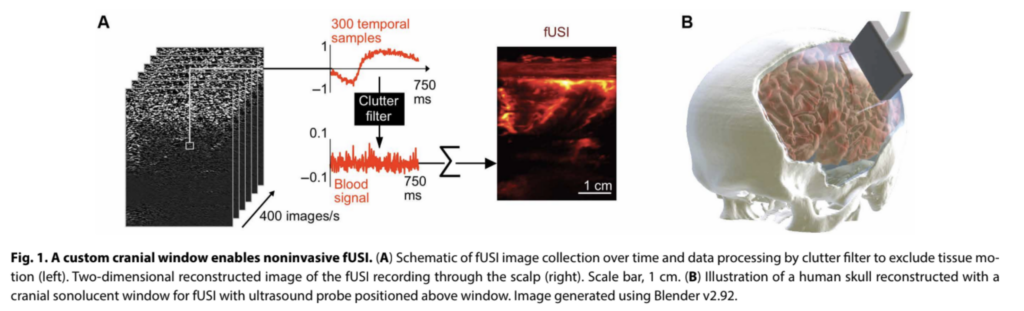
Optimizing acoustic window implants for humans
Partial removal of the human skull, and its permanent replacement with another material, is a fairly common procedure, used as part of interventions to relieve high intracranial pressures resulting from injury, stroke or hemorrhage.
Starting from this point, the authors investigated materials that would provide the best performance for fUS imaging. Using a rat model, they compared the performance of two FDA-approved materials, poly(methyl methacrylate) (PMMA) and titanium mesh, finding that a 2 mm thick window of PMMA provided the best trade-off between fUS image quality and window robustness.
Into the clinic with fUS
What might this research mean for the future of fUS-assisted clinical interventions?
The authors point out that current methods for monitoring anatomical and functional brain recovery after brain surgery are difficult and expensive to carry out. They go on to say that “in the future, fUSI and custom cranial implants with acoustic windows may enable routine monitoring during the postoperative period”. This, they say, may have particular value for monitoring early signs of, or recovery from, pathologies that can develop following brain surgery, such as ‘syndrome of the trephined’.
We would also agree with the authors that the work is a significant step towards the development of fUS-based brain–machine interfaces (BMIs) in humans – a topic that is receiving much interest from the academic community. This is because throughout the two studies mentioned above, the participant was not in a surgery environment, but simply sitting in a chair. This opens up the prospect of being able to monitor patients as they go about everyday tasks, greatly expanding the number of people who might be willing to put themselves forward for study, and therefore the pool of data needed for development of BMIs.
Bruno Osmanski, Ph.D., CTO and co-founder of Iconeus, concludes “this is a groundbreaking study in the field of fUS, and one that is likely to incentivize the more widespread use of acoustic windows in patients who need cranial surgery. Following on from the first clinical use of our Iconeus One system early in 2024, this shows that clinical applications of fUS are developing fast – and we hope and expect that benefits to patients will follow”.
Reference:
C. Rabut, S.L. Norman, W.S. Griggs, J.J. Russin, K. Jann, V. Christopoulos, C. Liu, R.A. Andersen and M.G. Shapiro, Functional ultrasound imaging of human brain activity through an acoustically transparent cranial window, Science Translational Medicine, 2024, 16: eadj3143, https://doi.org/10.1126/scitranslmed.adj3143.



